We would like to remind you that on 27 March 2025, a new law on dietary supplements was adopted (Law of Ukraine No. 4122-IX “On Amendments to Certain Laws of Ukraine Aimed at Improving the Regulation of the Manufacture and Circulation of Dietary Supplements and Addressing Other Issues in the Healthcare Sector”). The law entered […]
Perspective: MDR, UDI, and EUDAMED in Ukraine
As Ukraine transitions to MDR and IVDR, the government aims to use European electronic systems rather than developing its own. This approach seems reasonable, but is it feasible?
One of the key innovations of MDR and IVDR is the Unique Device Identification (UDI) system and the EUDAMED electronic database. In Ukraine, the regulation of medical devices is often modeled after pharmaceutical regulations. However, it is crucial to understand that UDI is not analogous to drug serialization, as it does not involve sales registration or number exclusion from the database. Similarly, EUDAMED is not EMVS (European Medicines Verification System) or EudraGMP, as it serves a different purpose and functionality.
The UDI (Unique Device Identification) system ensures the traceability of medical devices throughout their lifecycle, enhancing control over their safety, effectiveness, and quality while also combating counterfeiting. UDI consists of two parts:
- UDI-DI (Device Identifier) – a unique identifier for a specific model of a medical device. In the most widely used GS1 system, UDI-DI typically corresponds to the GTIN, which is broadly utilized across various industries, including retail, medical devices, and pharmaceuticals. Both Ukrainian and international manufacturers have already applied this barcode, which can be scanned at checkout.
- UDI-PI (Production Identifier) – includes variable information such as batch number, serial number, manufacturing date, etc.
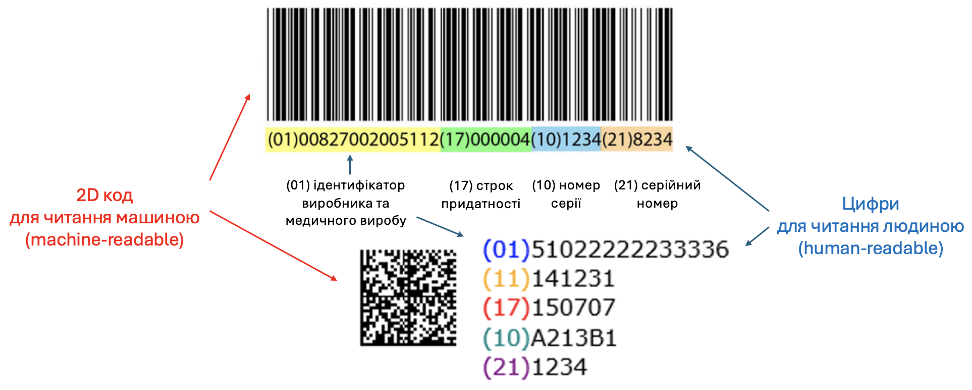
In the EU, manufacturers are required to register UDI in the relevant module of the EUDAMED system. Each device is linked to compliance documents (MDR/IVDR certificates and declarations of conformity), which are entered either by the manufacturer or the notified body (in the case of certified devices).
Economic operators—including manufacturers, authorized representatives, and importers, i.e., those placing medical devices on the market—must register in EUDAMED. They receive a unique registration number (SRN). Through the “device – certificate – operator” linkage, EUDAMED helps prevent the circulation of illegal products, counterfeit declarations, and fraudulent certificates.
Pharmacies, medical device stores, and other retail sellers are not required to register in EUDAMED, as medical devices can be sold in various locations, including supermarket checkouts or vending machines (e.g., condoms). Healthcare institutions are also not registered in EUDAMED unless they act as manufacturers, such as when re-sterilizing devices.
However, UDI is only a small part of the EUDAMED system, which contains much more information:
- Publicly accessible section: device names and descriptions, key UDI information, device classification, manufacturer and authorized representative details, certificate and declaration of conformity information, records of product recalls and safety measures, and basic information on clinical investigations.
- Restricted section (for regulatory authorities, notified bodies, and market operators): device serial numbers and expiration dates, safety data and incident investigations, technical documentation details, and regulatory decisions on non-compliance.
Information in EUDAMED is entered by European market operators themselves: EU notified bodies, manufacturers, authorized representatives, and importers. Each operator inputs their respective data, which is then linked to information from other participants.
Use of EUDAMED in Ukraine
EUDAMED is designed exclusively for residents of the European Union, participants of the common European market, and countries that have signed Mutual Recognition Agreements (MRAs). EU notified bodies upload data on MDR and IVDR compliance certificates, to which manufacturers link their medical devices. Manufacturers register European declarations of conformity, while non-EU manufacturers must associate their devices with authorized representatives and importers registered in the EU. EU regulatory authorities also use EUDAMED to manage safety measures, such as batch recalls and incident notifications.
A non-EU entity’s only role in EUDAMED is that of a manufacturer, who can only operate through an EU Authorized Representative. First, the authorized representative must register in the system, after which the non-EU manufacturer can submit information about their devices for the EU market.
It is highly unlikely that Ukrainian operators will gain access to EUDAMED before Ukraine joins the EU or signs the ACAA agreement, as the system is exclusively designed for the EU market. Currently, EUDAMED does not allow Ukrainian manufacturers, certification bodies, market surveillance authorities, or importers to input data, making such participation infeasible.
At the same time, implementing MDR and IVDR in Ukraine without a national equivalent of EUDAMED (even with limited functionality) is impractical. To align with the EU, Ukraine needs to introduce a UDI system and an economic operator registry because:
- Without digital identification of devices and operators, regulatory authorities will be unable to effectively monitor the market.
- The absence of a registry for certificates and declarations of conformity will complicate the verification of product legality.
- Non-compliance with MDR and IVDR traceability standards will hinder further integration into the EU common market.
Developing a Ukrainian equivalent of EUDAMED is a crucial step toward implementing MDR and IVDR analogs, harmonizing medical device regulations with EU legislation, and facilitating Ukraine’s future integration into the European common market.
Maksym Bagrieiev
Managing Partner

