Authorized representative in Ukraine
Prior to placing medical devices on the market of Ukraine, a non-resident Manufacturer must designate an Authorized Representative in Ukraine, which can be a legal entity or an individual entrepreneur that is a resident of Ukraine.
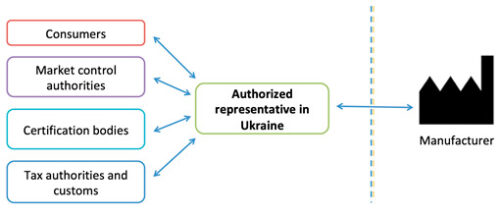
The Authorized Representative in Ukraine is the link between Ukraine (conformity assessment authorities, market surveillance authorities, revenue and collection authorities, consumers, etc.) and the Manufacturer, ensures the timely communications, and performs post-marketing surveillance.
Authorized representative
An Authorized representative – is any legal entity or individual entrepreneur who is a resident of Ukraine or registered in accordance with the legislation of Ukraine, a representative office of a foreign entity, that has duly confirmed the manufacturer’s authority to perform legal actions on it’s behalf regarding the manufacturer’s obligations established by the Technical Regulations.
The Authorized Representative has a lot of other rights and obligations: to keep the technical documentation accessible for competent authorities for at least 5 years (15 years for active implantable devices) from the moment of placing of the last medical device on the market, to participate in inspections of market surveillance authorities and to perform corrective actions on behalf of the Manufacturer, to perform post-marketing surveillance and to notify on incidents, to authorize importers, to blocks “gray” import etc.
Obligations and responsibilities are described in the Technical Regulations for medical devices, the Law “On State Market Supervision and Control of Non-food Products” and other legislative acts. The Order of the Ministry of Health of Ukraine No.142 dated January 22, 2020 approved the national guideline for Authorized representative, based on the European Commission’s Guideline MEDDEV 2.5/10 dated January 2012, but not legally binding.
The designation of an Authorized representative does not change the responsibility of the manufacturer. An Authorized representative must be designated and supervised by the manufacturer.
Each medical device (type/model) should be associated with only one Authorized Representative. The Manufacturer can designate several Authorized Representatives, but for different medical devices.
The Authorized representative’s name and location must be indicated on the labeling of each medical device and/or in the instructions for use (IFU). To improve communication with the consumer, we also recommend to indicate the contact phone number, email and website. The Authorized Representative’s name and address should be also indicated in the Declaration and Certificate of Conformity.
The Authorized representative can be an importer or distributor, or a third organization. Some manufacturers have an internal policy according to which an organization participating in the supply chain cannot be an Authorized representative. Likewise, if the Manufacturer works with several importers (distributors), the designation of any of them as the Authorized representative provides a significant competitive benefit, since its name and address will be indicated on the labeling of all products supplied to Ukraine.
Means of designation
The designation of the Authorized representative is carried out in writing by issuing a Power of Attorney and/or Agreement. The Power of Attorney should be duly certified and legalized.
We recommend to use both documents:
- The Power of Attorney has a smaller extent, it is convenient to use it for submission to external organizations, however, the Power of Attorney transfers rights only on a unilateral basis.
- The Agreement can regulate the rights and obligations of both parties, limit the liability of the Authorized Representative concerning the medical devices quality and safety, establish the terms of communication, the procedure for dealing with complaints and adverse event reports, and many other aspects.
Agreement on the designation of the Authorized representative is not equal to an import/distribution agreement and is usually a separate document, more similar in structure to the operational procedure. In some cases, the Agreement of the designation of the Authorized representative can be amended as an Annex to the import and distribution agreement.
Our lawyer can prepare a draft of the Power of Attorney and/or Agreement for the designation of an Authorized representative in Ukraine, answer the Manufacturer’s questions, explain the requirements for legalization and follow up the signing. We monitor legislation updates and important inquiries of the competent authorities (customs, market surveillance and conformity assessment authorities).
Registration of the Authorized Representative
In some cases, the data on the person responsible for placing the medical devices on the market must be notified (registered) to the competent authority, i.e. the State Service of Ukraine on Medicines and Drugs Control.
Registration is carried out before putting into circulation of the following products:
- Class I medical devices, including sterile devices and/or devices with measuring functions;
- Procedural kits;
- All medical devices for in-vitro diagnostics.
The procedure for registration is described in the Technical regulations and in the Order No. 122 of the Ministry of Health of Ukraine dated February 10, 2017. The Register is published in electronic form on the website of the State Service of Ukraine on Medicines and Drugs Control.
Duties and responsibilities
The duties of the Authorized Representative are described both in the Technical Regulations on medical devices and in related legislative enactments. The list of main responsibilities is as follows:
- Be responsible for the medical devices introduced into circulation.
- Interact with market surveillance authorities, law enforcement authorities and other competent authorities on behalf of the Manufacturer.
- Keep the documents accessible for competent authorities for at least 5 years (15 years for active implantable devices) from the date of placing of the last medical device on the market.
- Perform post-marketing surveillance:
- Collect data on incidents at the Ukrainian market and notify the Manufacturer,
- Submit to the competent authority data on all serious incidents in Ukraine and worldwide that could lead or led to the user’s death or significant health deterioration,
- Reveal technical or medical reasons that lead to systematic product recalls.
- Perform corrective and restrictive actions concerning the products.
- Inform the competent authority on corrective actions and product recalls.
- Pay fines for the introduction of non-conforming products.
Fines are established by Article 44 of the Law of Ukraine “On State Market Surveillance and Control of Non-Food Products”:
- Putting into circulation the products posed a serious risk: UAH 102,000.
- Putting into circulation the products that do not meet the established requirements: UAH 51,000.
- Failure to comply or incomplete implementation of the decision on taking restrictive (corrective) measures in relation to products posing a serious risk: UAH 170,000.
- Failure to comply or incomplete implementation of the decision on taking restrictive (corrective) measures in respect of other products: UAH 102,000.
- Obstructing by preventing inspections of the product characteristics: UAH 170,000.
Fines are imposed for each model, article or batch of the products that are dangerous, pose a risk and/or do not comply with the established requirements, regardless of the number of units of such products and/or places of sale.
Outsourcing of the Authorized representative
If a manufacturer, for some reason, cannot designate it’s importer (distributors) as an Authorized representative in Ukraine or intends to ship products to several distributors – Cratia can be contracted for that role. LLC “Cratia Medtekhnika” is a specialized legal entity performing functions of the Authorized representative to foreign manufacturers. Company is ISO 9001:2017 certified by Bureau Veritas (Italy), has huge experience and a strong team of competent professionals, provides 0-800 toll free telephone etc.
We are not involved in distribution processes that guarantee the absence of conflicts of interest and a highly professional approach. Our liability to third parties is insured under the Voluntary Professional Liability Insurance Agreement.
Dedicated website: https://uarep.com/
To start cooperation or to consult, you may contact us:
- by phone +38 044 361-48-28, +38 044 221-71-29,
- by e-mail info@cratia.ua,
- or arrange a meeting in our office.

