Essential information on certification
Starting from July 1, 2017 medical devices may be placed on the market and/or put into service only if they comply with the requirements of Technical regulations of Ukraine. In practice this means that at the time of import, sale, bringing into service or use of the medical devices, there must be:
- UA Declaration of conformity;
- UA Certificate of conformity and/or registration at the competent authority;
- Package labeling in the Ukrainian language with the symbol of conformity;
- Instructions for use in the Ukrainian language, if provided by the manufacturer;
- UA Technical documentation demonstrating compliance with the UA Essential Requirements.
Technical regulations define the routes (procedures) how the conformity assessment can be achieved and demonstrated. Applicable conformity assessment routes (procedures) depend on the class (potential risk associated with the technical design and manufacture) of the medical devices. The safest medical devices are subject to a fast-track simplified procedure, and the higher the risk, the higher the requirements and the more complicated procedure of certification.
According to Article 193 of the Tax Code of Ukraine, all medical devices that comply with the requirements of Technical Regulations are subject to a 7% VAT rate at import and circulation. For the period of quarantine caused by coronavirus disease, exemptions for the import and taxation for certain medical devices were introduced.
| Since 2007, we are providing professional consulting on conformity assessment (certification, registration) of medical devices in Ukraine. We have certified medical devices from more than 900 foreign and national manufacturers. Our specialists are proficient in local and EU legislation, communicate in professional English, possess large experience. By starting cooperation with us you receive a high-quality professional result. |
How to start conformity assessment (certification, registration) of medical devices?
Depending on the characteristics of the medical device, it is required to determine the applicable certification routes (procedures) and select the most suitable of them. There are several basic conformity assessment procedures:
- Self-declaration and registration in the State Service of Ukraine on Medicines and Drugs Control;
- Conformity assessment via an audit of manufacture;
- The accelerated and simplified procedure with partial recognition of the EU certificate;
- Per-batch certification.
The procedure is determined by the class of the medical device and its other characteristics (e.g., sterility, presence of measuring functions, the ancillary pharmaceutical substances, etc.). Quite commonly, you can choose the most convenient and cost-efficient procedure from several options.
The correctly chosen procedure can spare a lot of time and money, so it is important to analyze all possible options at the very beginning. Thus we usually start our cooperation by reviewing the list of products and some initial documents.
Conformity assessment of medical devices of higher classes is performed by 11 national conformity assessment bodies (CAB’s, analogs of EU notified bodies) designated for Technical regulations on medical devices. Each conformity assessment body has its own specialization, experts with different knowledge and skills, technical base, and different approach to the timing and costs of works. To make the right decision you need to be well versed in their capabilities, requirements and prices – the same things can be done quickly and cheaply in one authority and be complicated and expensive task in another one.
| We are proficient in all certification procedures and cooperate with all conformity assessment bodies. We can perfrom the benchmarking and compare the terms, calculate the costs of the initial procedure and annual maintenance (if any), inform you on all possible pitfalls and help you to avoid them. |
What is the Authorized Representative of manufacturer in Ukraine?
One of the first steps in the certification procedure is the designation (appointment) of the Manufacturer’s Authorized Representative in Ukraine. The Authorized Representative is a resident company, including a representative office of a foreign company, or an individual entrepreneur authorized in writing by a foreign Manufacturer to fulfill the required legislative requirements and resolve various issues in Ukraine.
The Authorized Representative’s name and address should be placed on the labeling of each medical device and/or in the instructions for use, and also indicated in the Declaration of Conformity and Certificate of Conformity. The Authorized Representative is not required to be the importer, but can authorize other companies for the import and distribution.
The Authorized Representative acts as a link between Ukraine (conformity assessment authorities, market surveillance authority, revenue and collection authorities, consumers, etc.) and a foreign Manufacturer:
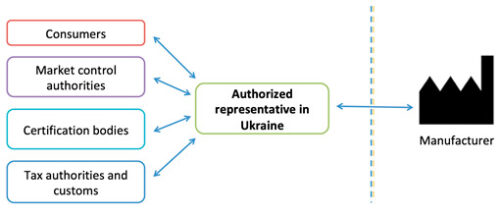
In addition, the Authorized Representative has many other obligations: it keeps technical documentation for at least 5 years, participates in inspections carried out by market surveillance authorities and pays fines for the Manufacturer, performs post-marketing surveillance and reports on incidents, blocks gray imports, etc.
Each medical device (type/model) should be associated with only one Authorized Representative. The Manufacturer can designate several Authorized Representatives, but for different medical devices.
To find more detailed information on the functions, rights and obligations of the Authorized Representative, please follow the link.
| Our legal department will prepare a draft Power of Attorney and/or Agreement for the appointment of the Authorized Representative in Ukraine, answer the Manufacturer’s questions, explain the requirements for legalization. We permanently monitor updates and respond in time to key remarks made by the customs, market surveillance and conformity assessment authorities. Please be assured that all legal documentation used in our work meets the most recent requirements of legislation and competent authorities. |
Documentation for conformity assessment of medical devices
It is required to collect, prepare and assmble the set of documents that shall demonstrate conformity with the essential requirements and to enable conformity to be verified. Set of documents should include the Manufacturer’s original Technical Documents and Ukrainian national documents and forms, decisions of the national conformity assessment bodies (if applicable). Documentaiton shall be kept by the Authorized representative or manufacturer at the disposal of the competent authorities for a period of at least 5 years (15 years for active implantable medical devices) from the date placing of the last medical device on the market of Ukraine.
Set of documents depend on the chosen conformity assessment route (procedure) and requirements of the conformity assessment body. Specific documents should be translated into Ukrainian, in particular, translation of the instructions for use (user manual) is mandatory.
Based on the Manufacturer’s documents, the following national documents should be prepared:
- Application for conformity assessment and/or notification for registration of a medical device;
- Instructions for use (if required);
- Labelling;
- Essential requirements check-list;
- Declaration of Conformity;
- Cover letters, etc.
National documents should be prepared in Ukrainian, and some of them can be bilingual for better understanding by the Manufacturer and simplification of customs clearance.

Mark of conformity to Technical Regulations
Technical Regulations and other legislative enactments stipulate the requirements for the forms and content of the documents, special national symbols etc. Follow the link to find more detailed information on the requirements for packaging labeling and instructions for use (user manual) for medical devices in Ukraine.
Based on this documentation, a conformity assessment is carried out, and the information is transferred to the certificate, Declaration, and Register.
Improperly prepared documents can cause the problems with customs clearance and loss of VAT preferences, denial of tenders, fines from market surveillance authorities, prohibition of circulation and other troubles.
This documentation also should be kept by the Manufacturer’s Authorized Representative for at least 5 years after putting the medical device into circulation. The market surveillance authority, i.e. the State Service of Ukraine on Medicines and Drugs Control, has right to check this documentation.
Documentaiton shall be kept by the Authorized representative or manufacturer at the disposal of the competent authorities for a period of at least 5 years (15 years for active implantable medical devices) from the date placing of the last medical device on the market of Ukraine. State market surveillance authorities (State Service of Ukraine on Medicines and Drugs Control, State Service of Ukraine on Food Safety and Consumer Protection) have the right to access the documents within that period.
| Our team is proficient in English, regulatory terminology, and the requirements of national and international legislation. Verification of compliance with the requirements of Ukrainian legislation, filling out national documents and forms is part of our work on the conformity assessment of medical devices. |
Simplified / fast-track procedure for certification of medical devices
Audit (inspection) of the manufacture is the most common, as well as the most time-consuming and costly procedure. Audit is mandatory for all sterile medical devices, all active implantable medical devices and is most commonly applicable for the devices of the III (maximum) class of potential risk, and “A” List devices for in vitro diagnostics.
The Certificate of Conformity is issued based on the audit results for the period of 5 years, but every 12 months it is required to carry out scheduled surveillance via abbreviated inspection of manufacture. Surveillance is a significant annual expenditure to maintain certification.
In February 2016, the Law of Ukraine “On Technical Regulations and Conformity Assessment” was adopted, which allowed to carry out simplified and accelerated procedure for medical devices approved in the European Union market.
This procedure is not an exchange of the EU certificate for the Ukrainian certificate. To carry out this procedure, it is required to meet all national requirements, including the designation of an Authorized representative in Ukraine, collecting of initial documents, filling of national documents and forms, fulfillment of the language and labeling requirements, following the steps of the chosen conformity assessment route.
However this procedure allows replacing of the audit from Ukrainian CAB’s by the assessment of the protocols of European notified bodies.
Recognition of the protocols of the European notification bodies allows avoiding all audits related to the conformity assessment of a medical device in Ukraine – initial, annual supervisory, recertification.
More detailed information on the procedure for recognizing EU certificates in Ukraine and the list of European authorities, certificates issued by which can be recognized in Ukraine.
 |
Please read our article “Recognition of EU certificates for medical devices in Ukraine” (October 2019). |
| We have carried out more than 500 certification procedures via simplified recognition procedure. We will be able to professionally and efficiently carry out an accelerated and simplified national conformity assessment procedure of a medical device. |
Registration of medical devices
Entering into the register of the State Service of Ukraine on Medicines and Drugs Control is a mandatory requirement for class I medical devices (including Is, Im classes), all devices for in vitro diagnostics and custom-made devices. These medical devices should be entered into the Register before placing on the market of Ukraine.
Registration is carried out in accordance with Order No. 122 of the Ministry of Health of Ukraine dated February 10, 2017; registration is performed upon submission of the set of documents submitted to the State Service of Ukraine on Medicines and Drugs Control. The Register is maintained in a publicly accessible electronic form on the website of the State Service of Ukraine on Medicines and Drugs Control.
Refusal to enter into the Register and exclusion from the register occurs as a result of submission of incomplete or unreliable data, missing the Representative or expiration of its term of office, non-compliance of medical devices with the established requirements, expiration of the Declaration of Conformity and/or the Certificate of Conformity.
Maintaining certification
Technical Regulations establish the requirements that must be met during the validity period of the Declaration of Conformity and/or Certificate of Conformity, as well as for 5 years after their expiration (15 years for active implantable devices).
Control over the fulfillment of post-marketing requirements is carried out by state market surveillance authorities and appointed conformity assessment authorities. Failure to comply with the requirements can lead to high fines (the minimum fine for an Authorized Representative is UAH 51,000), cancellation of VAT preferences or restrictions on the circulation of the products, suspension or cancellation of the Certificate and/or exclusion from the Register.
To start cooperation or to consult, you may contact us:
- by phone +38 044 361-48-28, +38 044 221-71-29,
- by e-mail info@cratia.ua,
- or arrange a meeting in our office.

