Labeling, language and notification
Cosmetic products placed on the market of Ukraine must be labeled. The requirements and the list of information that must be provided on the product packaging are established not only by the Technical Regulation on Cosmetic Products but also by about a dozen different normative legal acts.
According to the requirements of the Law of Ukraine “On Ensuring the Functioning of the Ukrainian Language as the State Language,” the language of labeling must be Ukrainian. Alongside the text in Ukrainian, information in other languages may also be provided. The use of abbreviations or symbols is also allowed.
The information must be visible, legible, and indelible, and it can be placed using a sticker, insert (leaflet), tag, or card.
Packaging layouts (graphic files and/or photographs) are subject to notification to the competent authority.
The Responsible person is responsible for ensuring that the labeling complies with legislative requirements. Each distributor (every entity in the supply chain, except for the manufacturer or importer) is required to verify the presence of the required labeling information before the product is made available on the market.
|
We provide professional services of market authorization (conformity assessment) of cosmetic products in Ukraine. We have deep knowledge of Ukrainian and European legislation, experience, and necessary resources. Our services:
Our team has an excellent knowledge of the legislation, is fluent in English, possesses broad experience, and will be able to answer all questions. Cooperation with us will grant you high-quality professional service and fast results. |
Information on the labeling
The Regulation specifies a list of mandatory information that must be indicated on the product packaging:
- Name and address of the Responsible person in Ukraine;
- Country of origin for imported products;
- Content of the packaging in units of weight or volume, or the number of product units;
- Date of minimum durability or period after opening;
- Storage conditions;
- Warnings and precautions;
- Special information for professional use;
- Batch number or identifying information;
- Product intended purpose, if it is not obvious;
- List of ingredients (composition) using INCI.
If the cosmetic product contains ingredients, colorants, preservatives, or UV filters listed in Annexes 3-6 of the Technical Regulation, relevant conditions and warnings must be included on the labeling.
Additional labeling requirements are established by the Law of Ukraine “On Consumer Rights Protection,” and for certain types of products, such as aerosol products, children’s cosmetics, etc., specific requirements are set by regulatory acts.
The labeling may include information stating that the product and its ingredients have not been tested on animals.
The legislation regulates not only mandatory but also voluntary information, including claims about the product.
Product claims
Claims can be in the form of texts, names, trademarks, photographs, images, or other marks that explicitly or implicitly provide characteristics or functions of the product on the labeling, in advertising, and marketing of cosmetic products.
These claims can relate to the product’s effectiveness, its ingredients, the product’s aesthetics (appearance, fragrance, shape, including packaging), consumer perception, or sensory properties during use, as well as comparisons with other products.
The Regulation requires that cosmetic product labeling must not contain claims that could suggest the product has characteristics or properties it does not possess. Thus, claims must be supported by reliable, relevant, and clear evidence and properly substantiated.
Such evidence can be based on generally recognized data, published reports, or experimental studies. The evidence package can consist of any one or a combination of several of these types of evidence, depending on the circumstances. The decision on how to provide this evidence should be made according to the nature of the product and the type of claim.
Supporting information for each claim made about the product must be included in the product information file and kept by the Responsible Person as part of their obligations under the Regulation.
Overall, claims about cosmetic products must be clear, truthful, objective, understandable, and provide sufficient information to enable consumers to make informed decisions.
Exaggerated, false, or unsubstantiated claims may be grounds for action by market surveillance authorities and/or the Antimonopoly Committee.
Symbols for cosmetic product labeling
The Technical Regulation establishes graphical symbols that allow information to be provided without duplicating it in multiple languages:
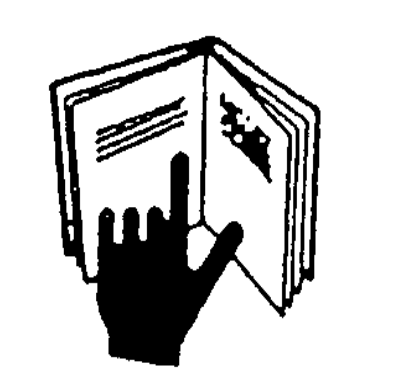
See information in accompanying materials |
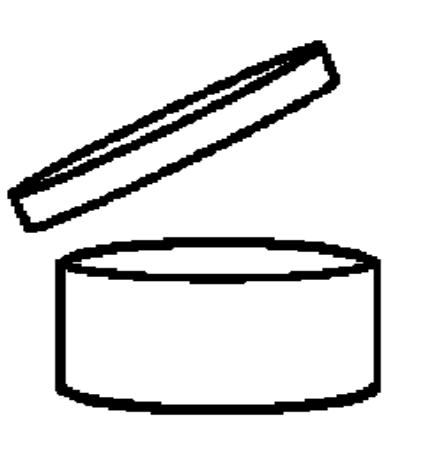
Period after opening |
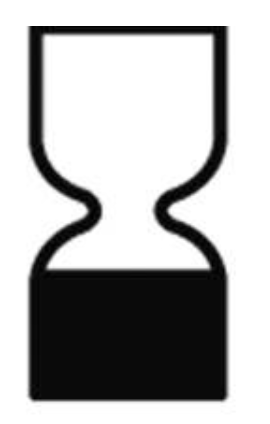
Minimum durability date |
Units of measurement
Units of measurement on cosmetic product labeling must be displayed using the International System of Units (SI), in accordance with the requirements of the Ministry of Economic Development’s Order No. 914 dated August 4, 2015.
Notification of packaging layouts (graphic files)
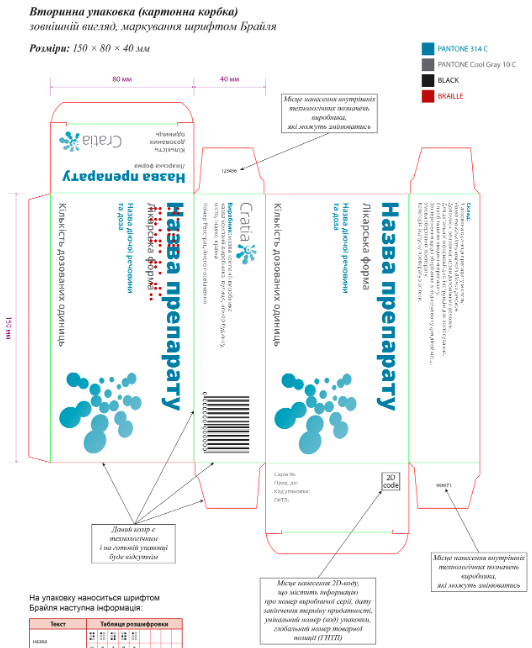 |
When placing cosmetic products on the market, the Responsible Person must notify (register) the graphic file of the labeling and, if necessary, a photograph of the packaging. The competent authority responsible for registering graphic files is the Ministry of Health of Ukraine. |
Our services
Cratia offers comprehensive regulatory support and assistance throughout all stages of placing cosmetic products on the Ukrainian market:
- Regulatory intelligence, consulting and feasibility study;
- Outsourcing of the Responsible person in Ukraine;
- Filling of the Product Information File (PIF);
- Filling of the Cosmetic Product Safety Report (CPSR) and Ukrainian safety assessor;
- Review and/or development of labeling, instructions, and advertising materials;
- Notification (registration) of the cosmetic product on the portal;
- Development of GMP according to ISO 22716;
- Compliance with UA CLP and UA REACH regulations;
- Compliance with the Technical regulation on aerosol dispensers;
- Legal and consulting support for manufacturers, responsible persons, or distributors (importers).
We possess the necessary knowledge and experience to conduct these tasks, and we are fluent in spoken and written English. We will manage and organize the process, help compile the necessary set of documents, and carry out the procedure within a short timeframe.
To start cooperation or to consult, you may contact us:
- by phone +38 044 361-48-28, +38 044 221-71-29,
- by e-mail info@cratia.ua,
- or arrange a meeting in our office.

