We inform you about key updates in the field of registration of medicines, quality control, and pharmacovigilance in Ukraine: Abolition of simplifications for GMP confirmation during the quality control of imported medicinal products; Implementation of eCTD; Adoption of the Law on Medical Cannabis; Scheduled and targeted audits of the Marketing Authorization Holder’s pharmacovigilance systems. Expert […]
Extension of transition periods and recognition of MDR and IVDR certificates in Ukraine
Cratia participated in the 12th Ukrainian Forum of Medical Device Market Operators, acting as an event sponsor and sharing important and current information about assessing the conformity of medical devices in Ukraine. The topic of the presentation was “Extension of recognition of MDD CE certificates until the end of 2028. Recognition of MDR and IVDR certificates in Ukraine”.
Viktoriia Moroz, a Key Account Manager, addressed a series of important issues regarding changes in the regulatory environment, namely the extension of recognition of EC MDD certificates until 2028 due to the extension of transition periods in the EU and the recognition of MDR and IVDR certificates in Ukraine.
Extension of recognition of MDD CE certificates until the end of 2028
In the first part of the presentation, Viktoriia emphasized that the recognition of EU certificates is a transparent and common way of assessing conformity. Recognition allows avoiding audits, which can be stressful for manufacturers, as it requires proper preparation, organization, and coordination of the entire process.
This year, changes in the European Union affected the transition period for MDR due to various conditions, including stringent MDR/IVDR requirements and tight compliance deadlines, the impact of the COVID-19 pandemic on clinical trials of medical devices, remote audits, and disruptions in global supply chains of medical devices.
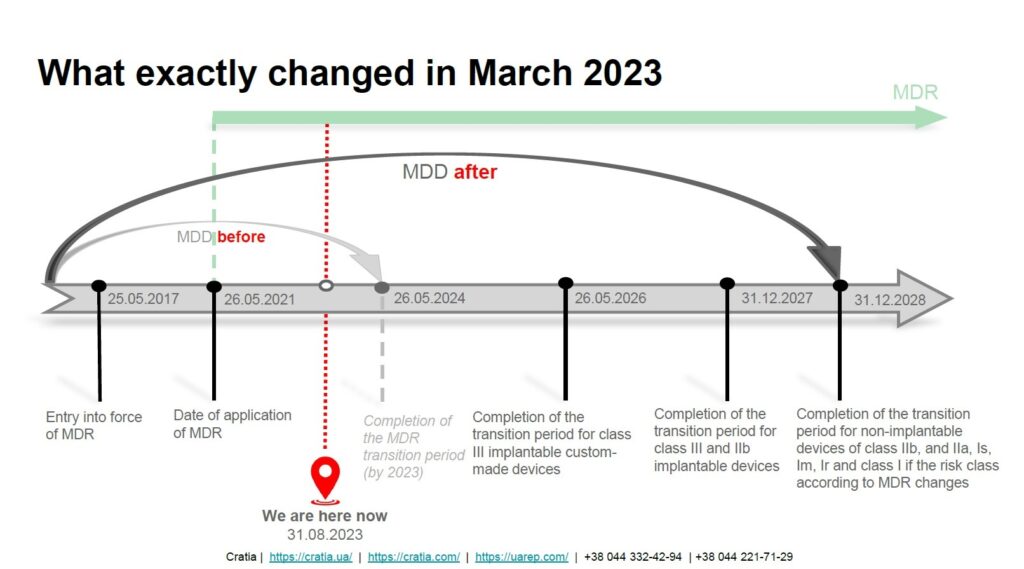
Previously, the transition period from MDD to MDR was set to end on May 26, 2024, and most EU certificates were valid until that date. The changes introduced by Regulation (EU) 2023/607 dated March 20, 2023, extended the validity of EC MDD certificates to new terms.
The new terms for transition periods are as follows:
- until 26 May 2026 for Class III custom-made implantable devices;
- until 31 December 2027 for Class III devices and Class IIb implantable devices excluding Well-established technologies (WET – sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips and connectors);
- until 31 December 2028 for other Class IIb devices, Class IIa, and Class I devices placed on the market in sterile condition or have a measuring function;
- until 31 December 2028 for devices not requiring the involvement of a notified body under MDD but requiring it under MDR (e.g., class I devices that qualify as reusable surgical instruments).
Changes in transition periods in the European Union also allow for the extension of the validity of Ukrainian certificates of conformity until December 31, 2028, or earlier, depending on the classification of medical devices. Furthermore, it is possible not only to extend existing certificates in accordance with Technical Regulation 753 but also to conduct initial certification by recognizing the MDD certificate with an extended validity period until 2027/2028.
Recognition of MDR and IVDR certificates in Ukraine
In the second part of the presentation, Viktoriia focused on the recognition of MDR and IVDR certificates, provided legislative grounds for the recognition procedure in Ukraine, and shared practical advice, leading to an active discussion with the audience.
The requirements of the new MDR and IVDR Regulations are higher than the previous requirements of the MDD and IVDD Directives. Consequently, the level of requirements of MDR and IVDR is higher than Ukrainian technical regulations for medical devices.
According to Article 45, Paragraph 1 of the Law of Ukraine “On Technical Regulations and Conformity Assessment,” recognition and acceptance of conformity assessment results conducted in another country are possible if the level of conformity to requirements is the same or higher. Therefore, as a conclusion, the recognition of MDR and IVDR certificates in Ukraine is possible and, in many aspects, considered the most reasonable option, as emphasized by Viktoriia.
None of the requirements from the old MDD or IVDD Directives have been canceled in the new MDR or IVDR Regulations. In fact, MDR and IVDR establish additional requirements for conformity, safety, and control of medical devices.
The presentation highlighted important features of the procedure for recognizing MDR/IVDR certificates. Practical examples were provided, emphasizing what to pay attention to, what challenges to expect, what knowledge is required, and what efforts need to be made to recognize MDR/IVDR certificates in Ukraine. In particular, it is necessary to involve a significant amount of effort in preparing a checklist for compliance with the requirements of Annex 1 of Technical Regulation 753. This is because the provisions of the new EU checklist, General Safety and Performance Requirements (GSPR, formerly ERC), significantly differ from the provisions of the requirements of Annex 1 of Technical Regulation 753. It is worth noting that the checklist is one of the first documents requested by market surveillance authorities during inspections.
Additionally, labeling and instructions for use according to MDR/IVDR can be much more extensive, as the requirements for these documents have also undergone significant changes. Viktoriia reminded that labeling and instructions for use of medical devices in Ukraine must be in the state language in accordance with the requirements of the Law of Ukraine “On Supporting the Functioning of the Ukrainian Language as the State Language” (Paragraph 6, Article 33).
Therefore, the recognition of MDR and IVDR certificates is possible. This path of conformity assessment is transparent, faster, and more economical compared to audits. Additionally, the recognition procedure promotes cooperation and knowledge exchange between Ukrainian and European notified bodies. Implementing European practices in Ukraine is an important prerequisite for signing the ACAA Agreement, which is essentially an “Industrial visa-free” agreement with the EU regarding medical devices.

