Responsible person in Ukraine
Only cosmetic products for which a responsible person has been appointed can be placed on the market in Ukraine.
The responsible person is a legal or natural person residing in Ukraine who ensures that the cosmetic product complies with the requirements of the Technical Regulation, including compliance with composition, labeling, manufacturing in accordance with GMP requirements, preparation of the safety report, notification of the cosmetic product and packaging graphic file, keeping the product information file available for market surveillance authorities and updating it, monitoring adverse effects, and more.
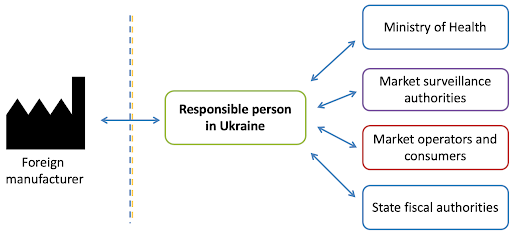 By default, the responsible person for domestically produced cosmetic products is the manufacturer, and for foreign-produced products, it is the importer. The national manufacturer and importer may appoint a third party to perform the functions of the Responsible Person by providing written authorization to a natural or legal person to act as the Responsible Person, provided that the latter gives their written consent to do so.
By default, the responsible person for domestically produced cosmetic products is the manufacturer, and for foreign-produced products, it is the importer. The national manufacturer and importer may appoint a third party to perform the functions of the Responsible Person by providing written authorization to a natural or legal person to act as the Responsible Person, provided that the latter gives their written consent to do so.
|
We offer the service of the responsible person in Ukraine. We have excellent knowledge of Ukrainian legislation in technical regulation of cosmetic products, have a strong and experienced team, and a robust quality management system. Cooperation with us will facilitate your business:
|
Agreement and Power of Attorney
The appointment of the Responsible Person is made in writing through a Power of Attorney. In addition to the Power of Attorney, an Agreement between the manufacturer and the Responsible Person can be signed, which more fully regulates the rights and obligations of both parties, sets communication deadlines, the procedure for handling complaints and reports of adverse effects, and other aspects.
Our legal department has developed exclusive drafts of the Power of Attorney and the Agreement for appointing the Responsible Person in Ukraine. We continuously monitor updates and respond promptly to key comments from customs, market surveillance authorities, and other competent bodies.
Obligations and responsibilities
Duties and Responsibilities The Responsible Person ensures compliance with the Technical Regulation on cosmetic products and is responsible for its fulfillment.
The obligations of the Responsible Person include:
- Ensuring the product composition complies with the Regulation;
- Ensuring the labeling meets legislative requirements;
- Conducting product safety assessments (safety report);
- Developing and continuously updating product documentation (product information file);
- Complying with requirements for product sampling and analysis;
- Ensuring the manufacturing process complies with GMP requirements;
- Notifying product data to the competent authority;
- Notifying packaging layouts;
- Storing product documentation and ensuring its availability to the market surveillance authority for 10 years after the last batch of the product is placed on the market;
- Providing public access to information about the qualitative and quantitative composition of the product;
- Providing distributor information upon request by the market surveillance authority;
- Reporting serious adverse effects to the market surveillance authority;
- Taking corrective actions in case of product non-compliance (bringing into compliance, market withdrawal, recall from sale);
- Interacting with government bodies, market operators of cosmetic products, and consumers in various situations.
The name and location of the Responsible Person must be indicated on the labeling of each cosmetic product.
Fines established by Article 44 of the Law of Ukraine “On State Market Surveillance and Control of Non-Food Products”:
- Introducing products into circulation that do not meet established requirements – up to 51,000 UAH (app. 1300 Euro).
- Introducing products into circulation that pose a serious risk – 102,000 UAH (app. 2600 Euro).
- Failure to implement or incomplete implementation of decisions on restrictive (corrective) measures regarding products posing a serious risk – 170,000 UAH (app. 3800 Euro).
- Failure to implement or incomplete implementation of decisions on restrictive (corrective) measures regarding other products – 102,000 UAH (app. 2600 Euro).
- Obstructing inspections by preventing the verification of product characteristics – 170,000 UAH (app. 3800 Euro).
Fines are imposed for each type, article, or batch of products that are dangerous, pose a risk, and/or do not meet established requirements, regardless of the number of such products and/or places of sale.
Outsourcing the Responsible person in Ukraine
Cratia provides the Responsible Person service in Ukraine. This allows you to:
- Delegate regulatory compliance and cosmetic product safety management to professionals;
- Separate the issues of compliance assessment from product supply/marketing;
- Collaborate with multiple distributors simultaneously without appointing any of them as the Responsible Person;
- Enhance the confidentiality of documentation.
We specialize in performing the functions of the Responsible Person, are well-versed in both Ukrainian and European legislation, have a large and well-coordinated team of professionals, and possess extensive experience in compliance assessment and related areas.
Our services
Cratia offers comprehensive regulatory support and assistance throughout all stages of placing cosmetic products on the Ukrainian market:
- Regulatory intelligence, consulting and feasibility study;
- Outsourcing of the Responsible person in Ukraine;
- Filling of the Product Information File (PIF);
- Filling of the Cosmetic Product Safety Report (CPSR) and Ukrainian safety assessor;
- Review and/or development of labeling, instructions, and advertising materials;
- Notification (registration) of the cosmetic product on the portal;
- Development of GMP according to ISO 22716;
- Compliance with UA CLP and UA REACH regulations;
- Compliance with the Technical regulation on aerosol dispensers;
- Legal and consulting support for manufacturers, responsible persons, or distributors (importers).
We possess the necessary knowledge and experience to conduct these tasks, and we are fluent in spoken and written English. We will manage and organize the process, help compile the necessary set of documents, and carry out the procedure within a short timeframe.
To start cooperation or to consult, you may contact us:
- by phone +38 044 361-48-28, +38 044 221-71-29,
- by e-mail info@cratia.ua,
- or arrange a meeting in our office.

